The Role of mRNA Vaccines in Modern Medicine
The advent of messenger RNA (mRNA) vaccines marks a pivotal shift in vaccinology, immunotherapy, and infectious disease control. Initially propelled into the spotlight during the COVID-19 pandemic, mRNA platforms are now being investigated across a spectrum of diseases, including cancer, autoimmune disorders, and rare genetic syndromes.
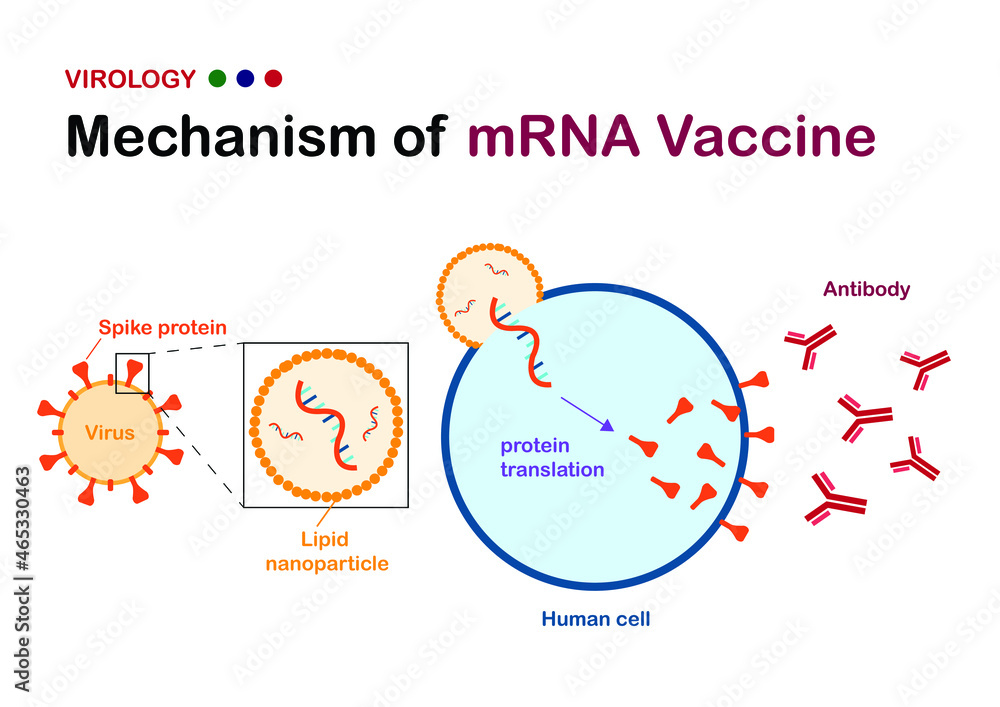
Mechanism of Action
Unlike conventional vaccines, which rely on weakened or inactivated pathogens, mRNA vaccines deliver a synthetic genetic blueprint encoding a specific viral protein. Upon cellular uptake, ribosomes translate the mRNA into protein antigens, typically the spike (S) protein in the case of SARS-CoV-2. These antigens are then displayed on the cell surface, eliciting both humoral and cellular immune responses.
“This paradigm leverages our own cellular machinery to produce antigenic material—an elegant fusion of biotechnology and immunology.” — Dr. L. Chen, Virologist, Harvard Medical School
Research published in Nature Medicine (2023) demonstrated that mRNA vaccines induced neutralizing antibodies and memory B cells exceeding levels observed in natural infection by over 5-fold.
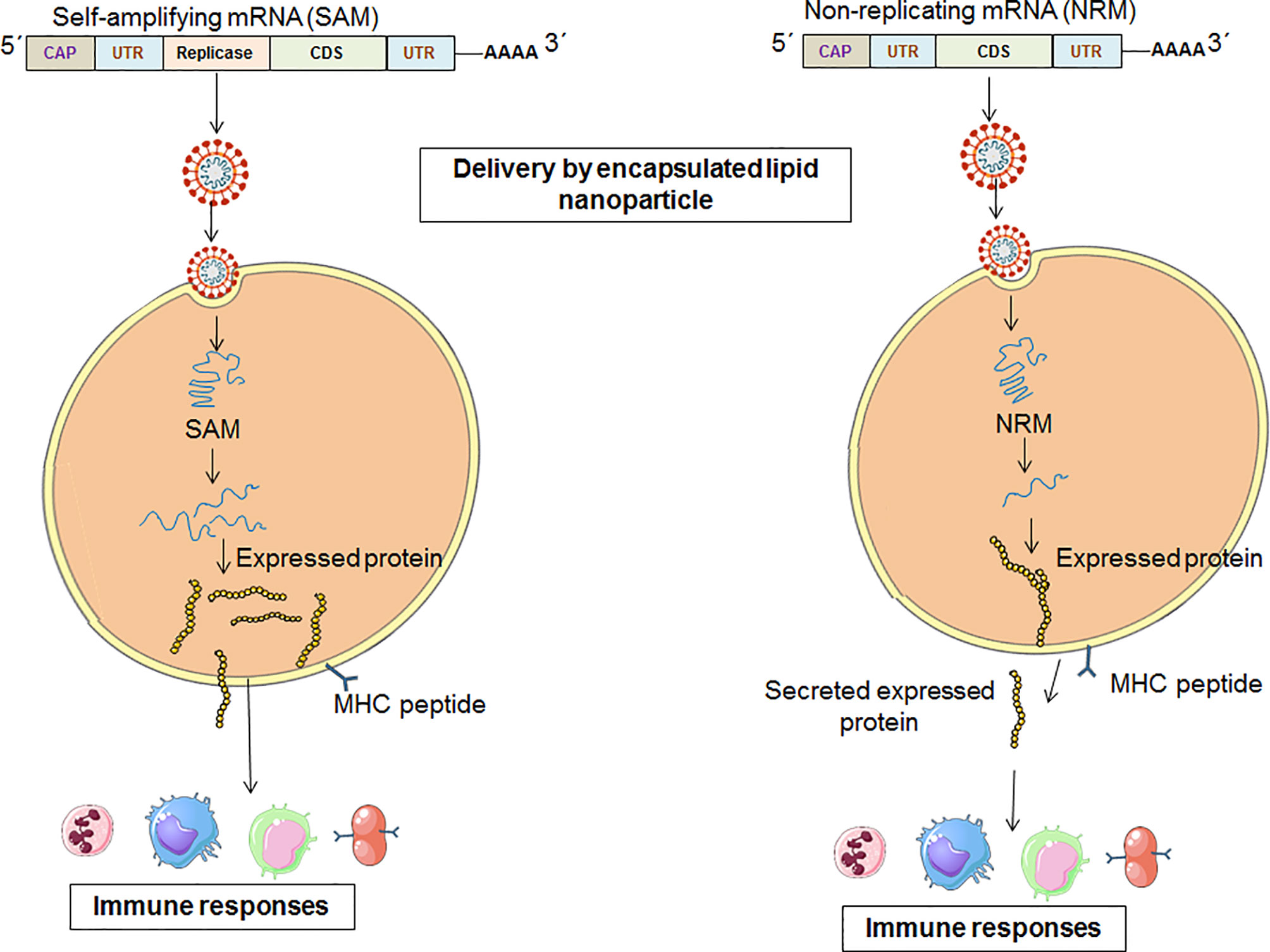
Advantages Over Traditional Vaccines
- Rapid design and scalability (within weeks of genome sequencing)
- Non-infectious and non-integrating platform
- Minimal allergenic components
- Potential for multivalent encoding (targeting several pathogens)
These properties make mRNA an ideal candidate not only for outbreak response but also for personalized oncology, where neoantigens unique to a patient’s tumor can be encoded and delivered.
Current and Future Applications
Clinical trials are ongoing for mRNA vaccines targeting:
- Influenza (Seasonal and Pandemic strains)
- Respiratory Syncytial Virus (RSV)
- Zika Virus
- Melanoma and non-small cell lung carcinoma (NSCLC)
A Phase I study from Moderna (NCT04536051) demonstrated favorable immunogenicity and tolerability profiles for mRNA-4157 in melanoma patients, showing early promise in adjuvant cancer therapy.
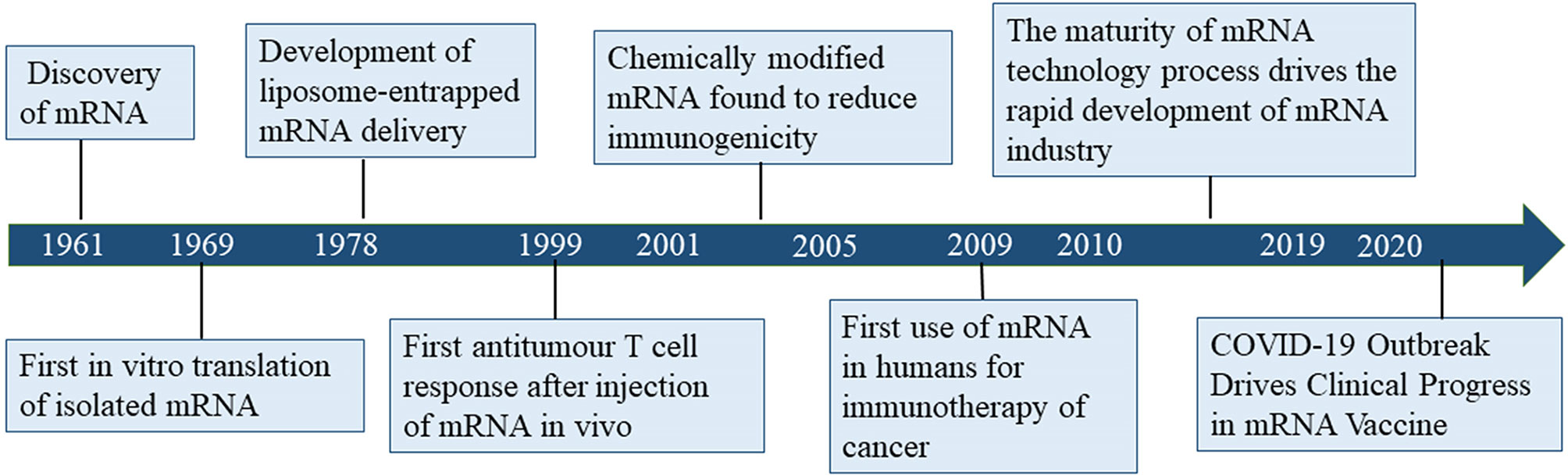
Challenges and Limitations
Despite their potential, mRNA vaccines face barriers related to cold-chain logistics, limited shelf life, and reactogenicity. Some individuals may experience systemic symptoms such as fever or fatigue, typically resolving within 48 hours.
Advances in lipid nanoparticle (LNP) stabilization and thermostable formulations are being explored to enhance global distribution equity—especially in low-resource settings.
“We are only beginning to understand how modular and adaptive mRNA platforms can be. This is the vanguard of precision vaccinology.” — Prof. Amina Rouhani, Institute of Biomedical Innovation← Back to Home